Patented, Unique in the market
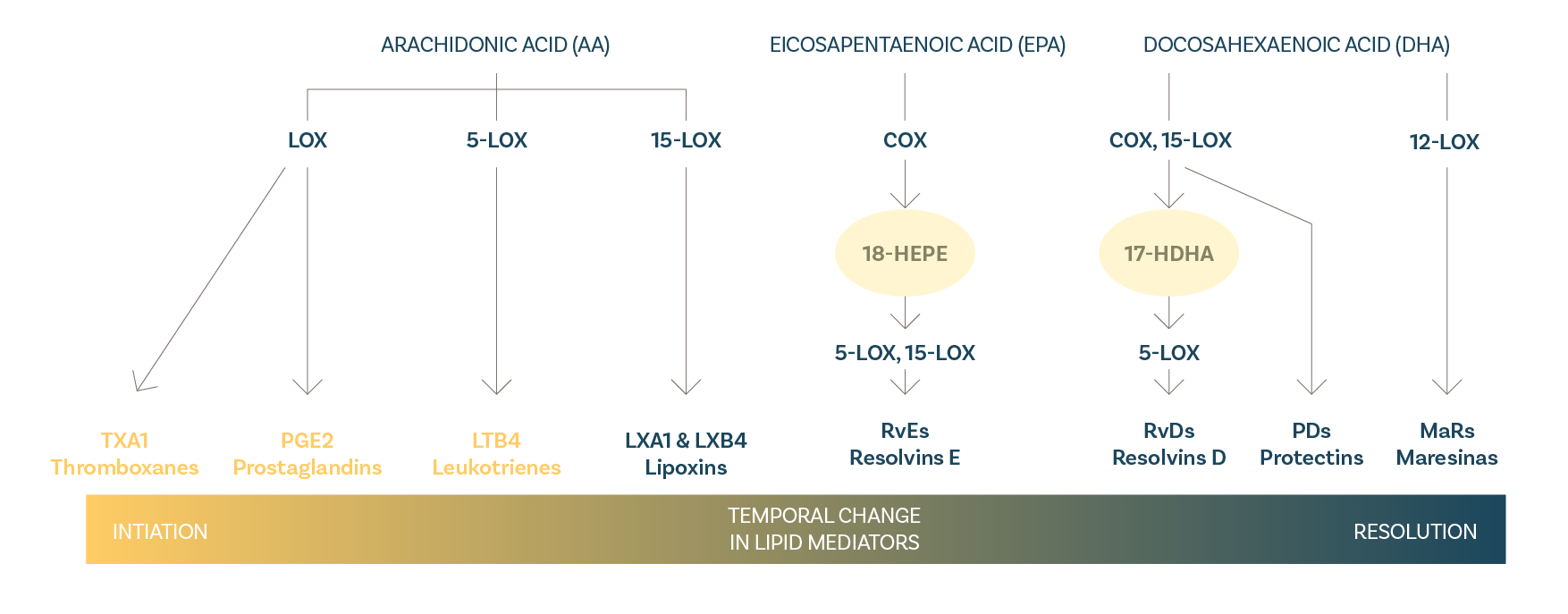
Standardised in pro-resolvis 17-HDHA + 14-HDHA + 18-HEPE
Vetilea Resolmega are capsules of Lipivet® specialized Omega-3 fatty acids from fish rich in DHA and EPA and standardized in pro-resolving mediators.
Pro-resolvins, active metabolites of DHA and EPA, help support the lipid metabolism and the immune system.
They help maintain the joints in good condition, contributing to mobility and flexibility and the quality of life of cats and dogs.
They contribute to a healthy immune system, especially in the skin, maintaining this organ in good health and restructuring the skin barrier.


Excellent palatability
No added flavourings
Comodo Format
60 easy-to-administer soft capsules
Omega-3 fatty acids have membrane structural function, and pro-resolvins are the endogenous metabolites in the fatty acid metabolite, of help in:
• In the skin, helping to restructure the skin barrier and supporting the skin immune system.
• In joints and muscles, helping to support mobility, flexibility and normal locomotor function.
• Supporting a normal immune response.
• In heart disease and renal patients as a support to accelerate recovery from inflammatory processes.
Fatty acid metabolic route